Nos expertises
Quel que soit le type d'engagement assistance technique, forfait, centre de service ou formation, nous sommes toujours orientés résultats et satisfaction.

12
Postes
disponibles
10
Agences
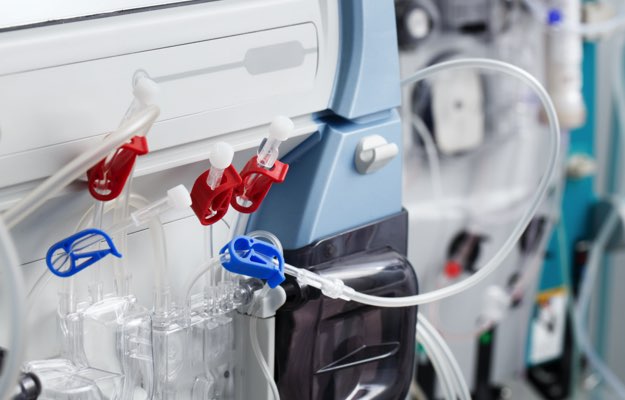
Sciences de la vie
Vos marchés
Les sciences de la vie sont des marchés dynamiques et porteurs, saisissez des opportunités grâce à notre expertise qui couvre vos principaux marchés.